General Chemistry
Explore the Foundations of Chemistry
Introduction to General Chemistry
General Chemistry serves as the foundation for understanding the principles that govern the physical and chemical behavior of matter. It explores the composition, structure, properties, and transformations of substances, providing learners with essential knowledge to navigate the natural and technological world.
This course is designed to develop both theoretical and practical skills essential for the fields of science, technology, engineering, and mathematics (STEM). Through interactive learning, experimentation, and real-world applications, students gain a deep appreciation of how matter behaves and interacts under various conditions.
The subject encompasses:
-
Matter and Its Properties (MP)
-
Measurements (MT)
-
Atoms, Molecules, and Ions (AM)
-
Stoichiometry (S)
-
Percent Composition and Chemical Formulas (PC)
-
Mass Relationships in Chemical Reactions (MR)
-
Chemical Reactions and Chemical Equations (CR)
-
Gases (G)
-
Dalton’s Law of Partial Pressures (DL)
-
Gas Stoichiometry (GS)
-
Kinetic Molecular Theory of Gases (KMT)
-
Electronic Structure of Atoms (ES)
-
Electronic Structure and Periodicity (ESP)
-
Chemical Bonding (CB)
-
Organic Compounds (OC)
-
Intermolecular Forces and Liquids and Solids (MF)
-
Physical Properties of Solutions (PP)
-
Thermochemistry (TC)
-
Chemical Kinetics (CK)
-
Chemical Thermodynamics (CT)
-
Chemical Equilibrium (CE)
-
Acid-Base Equilibria and Salt Equilibria (AB)

Matter and Its Properties: 1st Quarter Guide
Introduction
Matter makes up everything in the universe, from the air we breathe to the solid objects we touch. It is defined as anything that has mass and occupies space. The study of matter’s properties is fundamental in various scientific fields, including chemistry, physics, and engineering. By understanding how matter behaves and interacts, we can manipulate and apply it in practical ways, such as in manufacturing processes, environmental science, or medical technology. This guide will explore the Particulate Nature of Matter, the States of Matter, Physical and Chemical Properties, Extensive and Intensive Properties, Classification of Matter, and Separation of Mixtures, with a deeper dive into the unique characteristics of matter.
Key Topics:
1. Particulate Nature of Matter
Matter is made up of incredibly small particles, such as atoms, molecules, or ions, which are in constant motion. These particles are responsible for the observable characteristics of matter and govern its behavior under different conditions.
-
Characteristics:
-
Discrete Particles: The fundamental building blocks of matter are individual particles (atoms, molecules, or ions) that form various substances.
-
Constant Motion: Even in solids, particles vibrate. In liquids and gases, particles move more freely, increasing the rate of collisions and reactions.
-
Attractive Forces: The forces between particles vary in strength. Strong forces hold particles in solids, while weaker forces allow particles in liquids and gases to move.
-
-
Examples:
-
Water (H$_2$O): Water molecules are bonded together by hydrogen bonds. The properties of water, such as surface tension and boiling point, depend on these molecular interactions.
-
Table Salt (NaCl): Sodium and chloride ions in salt are held together by ionic bonds, which create a stable crystalline structure, affecting properties like solubility and melting point.
-
-
Applications:
-
In environmental science, the dispersion of pollutants such as gases or particles in the atmosphere can be explained using the kinetic molecular theory. This theory helps model air quality, pollutant spread, and the movement of microscopic pollutants in water.
-
2. States of Matter
Matter can exist in three primary states: solid, liquid, and gas. These states are defined by the arrangement, movement, and interactions of their particles. There are also plasma and Bose-Einstein condensates, but they are less common in everyday experiences.
-
Characteristics:
-
Solid: In a solid, particles are tightly packed and vibrate in place, leading to a fixed shape and volume. Solids have high density and are incompressible.
-
Liquid: Liquids have a definite volume but no fixed shape. Particles are close together but can move past one another, allowing liquids to flow.
-
Gas: Gas particles are far apart and move rapidly in all directions. Gases have neither fixed shape nor fixed volume, and they expand to fill their container.
-
-
Examples:
-
Solid: Ice has a rigid structure with water molecules arranged in a fixed pattern.
-
Liquid: Water has the ability to flow, but its volume remains constant.
-
Gas: Air is made up of gases like nitrogen, oxygen, and carbon dioxide, all of which are in constant motion and fill any available space.
-
-
Applications:
-
Plasma is used in technologies like fluorescent lamps and plasma TVs. Bose-Einstein condensates are studied in advanced physics, such as in research on quantum mechanics.
-
3. Physical and Chemical Properties of Matter
Understanding the physical and chemical properties of matter is fundamental in chemistry. These properties help us describe, identify, and distinguish different substances. Let's break down the two types of properties in more detail:
3.1. Physical Properties
Physical properties are characteristics of matter that can be observed or measured without changing the substance’s identity or chemical composition. These properties describe the physical characteristics of a substance, such as its appearance, texture, or state, without altering the chemical structure.
Characteristics of Physical Properties:
-
Can be observed without changing the substance: Physical properties can be identified through observation, measurement, or other techniques, without transforming the substance into something new.
-
Do not involve a chemical reaction: These properties pertain to the physical state and characteristics that remain the same regardless of chemical interactions.
Examples of Physical Properties:
-
Color: The color of a substance can be a helpful identifier. For example, copper sulfate is blue, while iron is metallic gray.
-
Density: Density is defined as mass per unit volume (e.g., g/cm³). For example, gold has a high density (19.32 g/cm³), and wood has a much lower density.
-
Melting and Boiling Points: The temperature at which a solid turns into a liquid (melting point) or a liquid turns into a gas (boiling point). For instance:
-
Ice melts at 0°C (273 K).
-
Water boils at 100°C (373 K).
-
-
Solubility: How well a substance dissolves in a solvent. For example, salt dissolves easily in water, while sand does not.
-
State of Matter: Matter exists in three common states: solid, liquid, and gas.
-
Solid: Fixed shape and volume (e.g., ice).
-
Liquid: Fixed volume but no fixed shape (e.g., water).
-
Gas: No fixed shape or volume (e.g., air).
-
-
Texture: How something feels to the touch. For example, the surface of metal is smooth, whereas the surface of sandpaper is rough.
-
Refractive Index: A measure of how much light bends when it passes through a substance. For example, glass has a specific refractive index that can be used in lenses and optical instruments.
-
Electrical Conductivity: How well a substance can conduct electricity. For example, copper is an excellent conductor of electricity, whereas rubber is an insulator.
Applications of Physical Properties:
-
Quality Control: The boiling point and density of liquids are often used to verify the identity and purity of chemicals in industrial applications.
-
Material Selection: The texture, color, and hardness of materials are important in choosing materials for construction, manufacturing, and art.
3.2. Chemical Properties
Chemical properties describe how a substance interacts with other substances to form new products. These properties are only observed during a chemical reaction, where the substance’s chemical composition is altered. Chemical properties are critical for understanding how substances react in various environments and how they behave during processes like combustion, corrosion, or metabolism.
Characteristics of Chemical Properties:
-
Involve a chemical reaction: Chemical properties can only be observed when a substance undergoes a chemical change (reaction), resulting in the formation of a new substance.
-
Change the substance's chemical composition: During a chemical reaction, the original substance is transformed into one or more different substances.
Examples of Chemical Properties:
-
Reactivity with Acids: Some substances react with acids to produce gases or other products. For example, zinc reacts with hydrochloric acid to form hydrogen gas and zinc chloride.
-
Flammability: This is the ability of a substance to burn in the presence of oxygen. For example, gasoline is highly flammable, while water is not flammable.
-
Oxidation: Oxidation occurs when a substance reacts with oxygen, often leading to rusting or corrosion. For example, iron reacts with oxygen and water to form rust (iron oxide).
-
Ability to Decompose: Some compounds break down into simpler substances over time. For example, hydrogen peroxide decomposes into water and oxygen when exposed to light or heat.
-
Reaction with Water: Some substances react vigorously with water. For example, sodium reacts with water to produce sodium hydroxide and hydrogen gas, which can be explosive.
-
Toxicity: The ability of a substance to cause harm to living organisms. For example, mercury and cyanide are toxic to humans and animals.
-
Acidity or Alkalinity (pH): The ability of a substance to act as an acid or base when dissolved in water. For example, vinegar is an acid, and ammonia is a base.
-
Electronegativity: The tendency of an atom to attract electrons in a chemical bond. For example, fluorine has a very high electronegativity, while sodium has a very low electronegativity.
Applications of Chemical Properties:
-
Industrial Processes: Chemical properties are essential in processes like combustion (e.g., burning of fuel), synthesis (e.g., creating compounds in the pharmaceutical industry), and metallurgy (e.g., extracting metals from ores).
-
Material Safety: Knowing the chemical properties of materials is crucial for safety in handling chemicals. For instance, flammability and toxicity are key factors in determining safe storage and use.
-
Corrosion Prevention: The oxidation property of metals is central to understanding how materials deteriorate over time, leading to innovations in rust-resistant coatings and alloys.
4. Extensive and Intensive Properties of Matter
In chemistry, properties of matter can be classified into two main categories: extensive and intensive properties. These classifications are important because they help in understanding how different properties behave when the amount of substance changes or remains constant.
4.1. Extensive Properties
Extensive properties are those that depend on the amount or quantity of matter present in a substance. These properties change when the amount of substance changes, meaning they are directly proportional to the size or mass of the sample.
Characteristics of Extensive Properties:
-
Depend on the quantity of matter: These properties change when the sample size changes.
-
Useful for quantifying material amounts: Extensive properties help in determining the total mass, volume, or energy content of a substance
Examples of Extensive Properties:
-
Mass: The amount of matter in a substance. For example, 1 kg of water has more mass than 0.5 kg of water.
-
Volume: The amount of space a substance occupies. For example, a large block of iron will have a larger volume compared to a small piece of iron.
-
Length: The measurement of how long an object is. For example, a 5-meter-long rod has a greater length than a 2-meter-long rod.
-
Total Energy: The total energy in a system depends on the amount of matter and its temperature. For example, a larger mass of water at 100°C will have more thermal energy than a smaller mass.
-
Weight: The force exerted by gravity on a substance. Weight is a function of both mass and the gravitational pull. For example, an object weighing 10 N on Earth would weigh much less on the Moon due to weaker gravity.
Applications of Extensive Properties:
-
Material Quantification: In industries, extensive properties are used to measure and control the quantities of materials. For example, knowing the mass and volume of a substance is important in manufacturing and packaging.
-
Energy Calculations: When calculating energy required for heating or cooling substances, the total mass and volume are crucial, such as in determining the energy needed to raise the temperature of a substance.
4.2. Intensive Properties
Intensive properties are those that do not depend on the amount of matter present. These properties remain the same regardless of the sample size, meaning they are independent of the quantity of substance.
Characteristics of Intensive Properties:
-
Independent of sample size: Intensive properties do not change when the quantity of the substance changes.
-
Useful for identifying substances: Intensive properties help in identifying materials or distinguishing between substances because they are characteristic of the material itself, not its quantity.
Examples of Intensive Properties:
-
Density: Density is the mass per unit volume of a substance. For example, the density of water is 1 g/mL, whether you have a small drop or a large volume.
-
Temperature: The temperature of a substance does not depend on the amount of material. A cup of water at 25°C will have the same temperature as a bathtub of water at 25°C.
-
Boiling and Melting Points: The temperature at which a substance boils or melts remains constant regardless of the amount of substance. For example, water boils at 100°C and melts at 0°C.
-
Refractive Index: This property measures how much light is bent as it passes through a material. The refractive index of glass is constant regardless of the size of the piece of glass.
-
Color: The color of a substance, like the green of copper sulfate or the yellow of sulfur, is an intensive property and does not depend on the quantity of the substance.
-
Hardness: The ability of a substance to resist scratching or deformation. For example, diamond has a higher hardness than graphite.
-
Electrical Conductivity: The ability of a substance to conduct electricity is independent of the quantity of the substance. For example, copper remains an excellent conductor, whether it’s in a wire, sheet, or small piece.
-
Magnetism: Some materials are magnetic, such as iron, and this property is independent of the amount of the substance.
Applications of Intensive Properties:
-
Material Identification: Intensive properties are essential for identifying materials. For example, the boiling point of a liquid can help differentiate between substances.
-
Quality Control: In manufacturing and industrial processes, intensive properties like density or melting point are used to ensure that products meet specific standards and specifications.
-
Environmental Science: In environmental science, intensive properties like temperature and density are used to understand the behavior of substances in different ecosystems or the atmosphere.
5. Classification of Matter
Matter can be classified based on its composition and structure. Understanding the classification of matter is essential for distinguishing substances, predicting how they behave, and applying this knowledge in various fields such as chemistry, biology, and environmental science.
Matter is typically divided into pure substances and mixtures, and these categories are further subdivided based on their properties.
1. Pure Substances
A pure substance consists of only one type of particle. These particles can be either individual atoms or molecules. Pure substances have constant and uniform properties throughout and cannot be separated into simpler substances by physical means. Pure substances are divided into two types:
a. Elements
-
Definition: An element is a pure substance that consists of only one type of atom. Elements are the simplest form of matter and cannot be broken down into simpler substances by chemical means.
-
Examples:
-
Oxygen (O): A gas that makes up 21% of Earth's atmosphere.
-
Gold (Au): A metallic element known for its malleability and conductivity.
-
Carbon (C): Found in various forms, including diamonds and graphite.
-
Characteristics of Elements:
-
Made up of atoms with the same atomic number (number of protons).
-
Each element has unique properties such as melting point, boiling point, density, and reactivity.
-
Can combine with other elements to form compounds.
-
-
b. Compounds
-
Definition: A compound is a pure substance composed of two or more different types of atoms chemically bonded together. The elements in a compound are present in fixed ratios, and the compound has distinct properties from those of the individual elements.
-
Examples:
-
Water (H₂O): A compound made up of hydrogen and oxygen atoms.
-
Sodium chloride (NaCl): Table salt, made of sodium and chlorine atoms.
-
Carbon dioxide (CO₂): A gas composed of carbon and oxygen atoms.
-
Characteristics of Compounds:
-
The properties of compounds differ from the properties of the individual elements that make them up.
-
Can only be separated into their components by chemical means, such as chemical reactions.
-
Have a fixed composition and can be described by a chemical formula.
-
-
2. Mixtures
A mixture is a combination of two or more substances that are not chemically bonded. The components of a mixture retain their individual properties and can usually be separated by physical methods. Mixtures can be categorized into homogeneous and heterogeneous mixtures based on their composition and appearance.
a. Homogeneous Mixtures (Solutions)
-
Definition: A homogeneous mixture is a mixture in which the components are uniformly distributed throughout, and it has a consistent composition. The individual components are not visibly distinguishable.
-
Examples:
-
Saltwater: A mixture of salt dissolved in water.
-
Air: A mixture of gases like nitrogen, oxygen, and trace gases.
-
Alloys: Mixtures of metals, such as bronze (copper and tin).
-
Characteristics of Homogeneous Mixtures:
-
Have a uniform composition throughout.
-
The components cannot be easily distinguished by the naked eye.
-
The properties are the same throughout the mixture.
-
Often exist as solutions (liquid), but they can also be gaseous or solid mixtures.
-
-
b. Heterogeneous Mixtures
-
Definition: A heterogeneous mixture is a mixture where the components are not uniformly distributed. The individual substances or phases are often visible and can be physically separated.
-
Examples:
-
Salad: A mixture of vegetables and dressing.
-
Sand and water: A mixture of solid sand particles in liquid water.
-
Oil and water: These do not mix, forming distinct layers.
-
Characteristics of Heterogeneous Mixtures:
-
The components are not evenly distributed throughout.
-
Individual components can often be seen and separated.
-
The properties can vary from one part of the mixture to another.
-
Often consist of two or more phases (e.g., liquid and solid).
-
-
3. Colloids
A colloid is a type of mixture where the particles are intermediate in size between those in a solution and those in a suspension. The particles in a colloid are small enough that they do not settle out over time but are large enough to scatter light. Colloids can exist in different phases (solid, liquid, gas), and their properties are unique compared to homogeneous and heterogeneous mixtures.
Examples of Colloids:
-
Milk: A colloidal suspension of fat globules in water.
-
Fog: Tiny water droplets suspended in air.
-
Gelatin: A solid colloid made by dissolving gelatin in water.
Applications of Classification of Matter:
-
Environmental Science: Understanding mixtures like air (a homogeneous mixture) and pollutants (heterogeneous mixtures) can help in air quality management.
-
Pharmaceutical Industry: Pure substances like compounds (e.g., drugs) are essential for medication formulation, while mixtures are often used in products like ointments or solutions.
-
Material Science: The classification of matter helps in the development of materials such as alloys (homogeneous mixtures) or composite materials (heterogeneous mixtures).
6. Separation of Mixtures
Mixtures can be separated using various physical methods, based on the differing properties of the substances involved.
Techniques for Separation of Mixtures
1. Filtration
Filtration is used to separate insoluble solids from liquids or gases based on particle size. A porous material (like filter paper or mesh) is used to trap solid particles while allowing the liquid or gas to pass through.
-
How It Works: The mixture is poured into a filter. The liquid or gas passes through the filter, while the solid particles are left behind.
-
Example: Separating sand from water, filtering coffee grounds from brewed coffee.
-
Application: Water purification, laboratory separation.
2. Distillation
Distillation separates liquids based on their differences in boiling points. This method works best for separating components that are in liquid form and have different volatilities.
-
How It Works: The mixture is heated to boil off the more volatile component, which is then condensed back into liquid form.
-
Example: Separating alcohol from water, separating crude oil into fractions.
-
Application: Purification of liquids, distilling water, industrial separation of chemical compounds.
3. Chromatography
Chromatography separates components based on their affinity for a stationary phase (e.g., paper, silica) and a mobile phase (e.g., solvent). It is used to separate substances in complex mixtures based on differences in their movement or solubility.
-
How It Works: The sample is applied to a stationary phase, and a solvent (mobile phase) moves through the material. The components of the mixture move at different rates and are separated.
-
Example: Separating different dyes in ink, purifying chemicals in laboratories.
-
Application: Forensic analysis, pharmaceutical applications, food analysis.
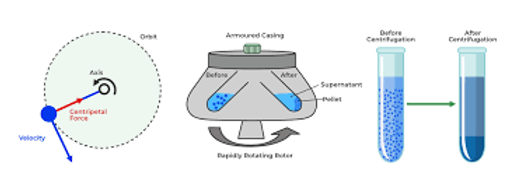
4. Centrifugation
Centrifugation uses rapid spinning to separate components of a mixture based on their density. The denser components move toward the bottom, while lighter ones remain at the top or suspended.
-
How It Works: The mixture is placed in a centrifuge tube and spun at high speeds. Components are separated due to centrifugal force.
-
Example: Separating blood plasma from red blood cells, separating cream from milk.
-
Application: Medical labs (blood tests), dairy processing, industrial separation.
5. Evaporation
Evaporation is used to separate a liquid from a dissolved solid by heating the mixture. The liquid evaporates, leaving the solid behind.
-
How It Works: Heat is applied to the liquid mixture until the liquid evaporates, leaving behind the dissolved solid.
-
Example: Evaporating water from saltwater to obtain salt, evaporating a solvent to obtain a dissolved substance.
-
Application: Salt extraction, concentration of solutions.
6. Magnetic Separation
Magnetic Separation is used when one component of the mixture is magnetic. A magnet is used to attract and separate magnetic materials from non-magnetic ones.
-
How It Works: A magnet is used to attract magnetic components from the mixture, leaving behind the non-magnetic materials.
-
Example: Separating iron filings from sand, removing metal contaminants from a mixture.
-
Application: Recycling, mining, food processing.
7. Sublimation
Sublimation is a process where a solid directly changes into a gas without passing through the liquid phase. This technique is used to separate substances that can sublime from those that cannot.
-
How It Works: The solid mixture is heated, and the substance that sublimes directly into a gas is separated from the other components.
-
Example: Separating iodine crystals from sand, purifying naphthalene (mothballs).
-
Application: Purification of volatile substances in chemistry.
8. Handpicking
Handpicking is a simple separation method used for large, easily visible components of a mixture. This method involves physically picking out different components by hand.
-
How It Works: Components are separated manually based on their size, color, or shape.
-
Example: Separating stones from rice, removing seeds from fruits.
-
Application: Food processing, small-scale separations in laboratories.
9. Sieving
Sieving is a method used to separate mixtures based on particle size. A sieve (a screen or mesh) is used to separate finer particles from coarser ones.
-
How It Works: The mixture is poured into a sieve. Smaller particles pass through the mesh, while larger particles are left behind.
-
Example: Separating flour from bran, separating gravel from sand.
-
Application: Construction (e.g., separating sand), food industry (e.g., separating different grain sizes).
10. Decantation
Decantation is a method used to separate liquids or solids from a mixture based on density. It is most commonly used when there is a clear difference in density between the components.
-
How It Works: The mixture is allowed to settle. The less dense liquid (or supernatant) is carefully poured off, leaving the denser solid or liquid behind.
-
Example: Pouring off clear liquid from a suspension of mud, separating oil from water.
-
Application: Wastewater treatment, separating liquids with different densities (oil and water).
11. Frosting / Freezing
Frosting or Freezing can be used for separating substances based on their freezing points. This method is mostly used in food processing or when dealing with compounds with different freezing points.
-
How It Works: The mixture is cooled, and the substance with a higher freezing point solidifies first, allowing separation.
-
Example: Freezing water to separate it from a syrup or solution.
-
Application: Separation of ice from salty water, crystallization in food industry.
12. Liquefaction
Liquefaction is a process that changes a gas to a liquid by increasing pressure or reducing temperature. This can be used to separate gases or liquefy them for storage or further processing.
-
How It Works: A gas mixture is cooled or pressurized until the gas condenses into a liquid, allowing separation from other gases.
-
Example: Separating carbon dioxide from air by liquefying it under pressure.
-
Application: Natural gas processing, air separation for oxygen production.